
Research Effectively Models Rotational TBI, Discovering Potential Neuroprotective Therapies

Each year, millions of people suffer traumatic brain injuries (TBIs) worldwide. Caused by an external force — such as a blow to the head that disrupts normal brain function — TBIs can often lead to a wide range of symptoms post-injury. Many patients have reported headaches, memory loss, difficulty with concentration and attention, mood changes and even disturbances to their sleep.
“These symptoms can be incredibly disruptive to a person's daily life, leading to decreased quality of life, difficulty at work and strained relationships with loved ones,” said James A. Bibb, PhD, chair of the University of Arizona College of Medicine – Phoenix Department of Translational Neurosciences.
Most TBIs are a result of falls, traffic accidents or sports-related head injuries — where the brain is damaged from the rapid acceleration/deceleration of the brain within the skull. Recreating these experiences in a controlled laboratory setting is very challenging. “Very few preclinical models of TBI recapitulate these negative rotational injuries. This has made it difficult to study the effects of TBI and to test potential treatments in a controlled environment,” said Dr. Bibb.
Yet a recent study by Umfress et. al — published in Scientific Reports and conducted in Dr. Bibb’s lab — highlights the development of a novel model of rotational acceleration-induced TBI.
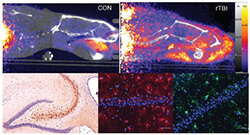
Utilizing complex mechanical engineering and high-speed telemetry, Dr. Bibb and his colleagues were able to engineer and characterize this novel model, which unveiled numerous neuropathological consequences of injury.
The investigators used translational in vivo imaging of resident immune cells to demonstrate diffuse neuroinflammation following rotational head trauma. Partnering with Cell Signaling Technology, they also utilized proteomic strategies to identify Cyclin Dependent Kinase 5 (Cdk5) as a principal perpetrator of TBI-induced memory impairments.
They coupled this with a novel inhibitor of Cdk5 — also discovered by Dr. Bibb’s lab — to demonstrate neuroprotection from those memory impairments and other neuropathological changes. Their progress provides foundational steps toward modeling clinically relevant forms of TBI, identifying biochemical alterations in the brain and developing novel neuroprotective therapeutics.
“Chronic effects of TBI include increased risk of developing neurodegenerative diseases — such as Alzheimer’s Disease and Parkinson’s Disease. And repetitive TBI can result in degenerative conditions such as chronic traumatic encephalopathy (CTE) — an affliction commonly observed in professional football players and other contact sport athletes,” Dr. Bibb explained. It is vital that researchers develop more effective therapies.
This multi-institutional study included collaborative contributions from the University of Texas at Dallas Erik Jonsson School of Engineering and Computer Science, the University of Texas Southwestern, the University of Alabama at Birmingham, Cell Signaling Technology and the U of A College of Medicine – Phoenix Department of Translational Neurosciences.
About the College
Founded in 2007, the University of Arizona College of Medicine – Phoenix inspires and trains exemplary physicians, scientists and leaders to advance its core missions in education, research, clinical care and service to communities across Arizona. The college’s strength lies in our collaborations and partnerships with clinical affiliates, community organizations and industry sponsors. With our primary affiliate, Banner Health, we are recognized as the premier academic medical center in Phoenix. As an anchor institution of the Phoenix Bioscience Core, the college is home to signature research programs in neurosciences, cardiopulmonary diseases, immunology, informatics and metabolism. These focus areas uniquely position us to drive biomedical research and bolster economic development in the region.
As an urban institution with strong roots in rural and tribal health, the college has graduated more than 1,000 physicians and matriculates 130 students each year. Greater than 60% of matriculating students are from Arizona and many continue training at our GME sponsored residency programs, ultimately pursuing local academic and community-based opportunities. While our traditional four-year program continues to thrive, we will launch our recently approved accelerated three-year medical student curriculum with exclusive focus on primary care. This program is designed to further enhance workforce retention needs across Arizona.
The college has embarked on our strategic plan for 2025 to 2030. Learn more.